half life formula for zero order reaction
Here k is the rate constant A is the preexponential factor Ea is the energy of activation R is the ideal gas constant and T is absolute temperature. Frac12A_0 k t_frac12 A_0 And rearranging.

Zero Order Reaction Definition Examples Formula
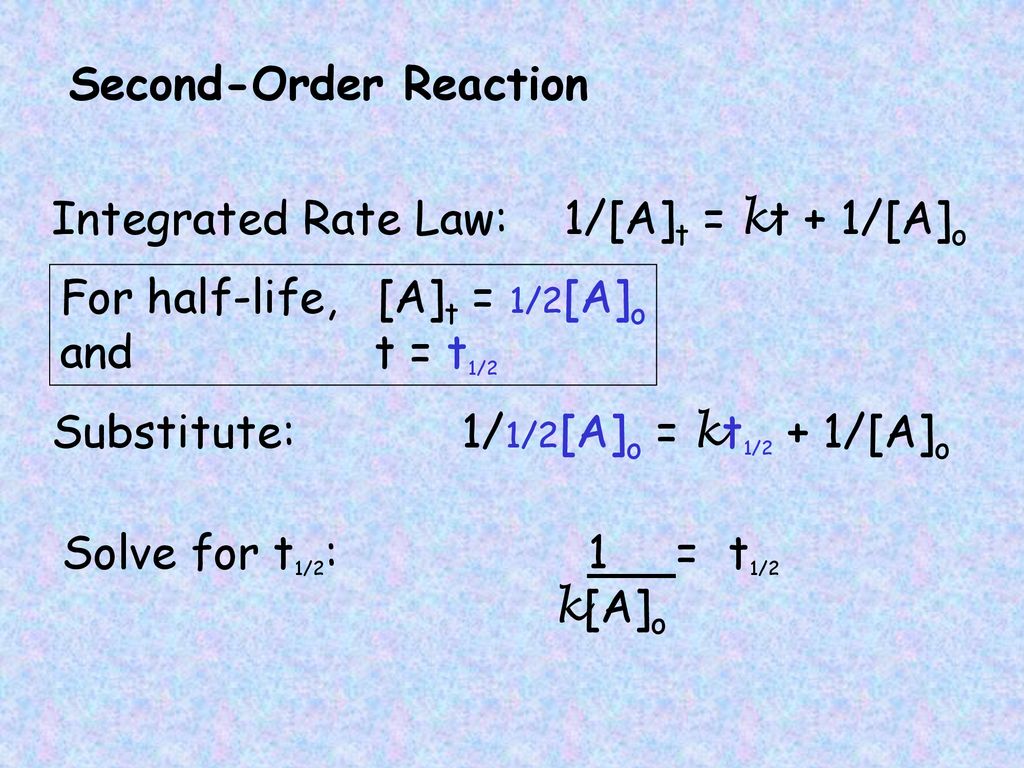
Therefore A2 k 0 t ½ or t ½ A2k.

. The half-life equation for a zero-order reaction is latext_frac12fracA_02klatex. For a zero order reaction A products rate k. The integrated rate law for the zero-order reaction A products is A_t -kt A_0.
The formula for half-life in chemistry depends on the order of the reaction. As the concentration decreases the half-life of the zero order reaction also decreases. What is the equation for the half-life of a zero-order process.
For a second-order reaction. Determining a half life. Because this equation has the form y mx b a plot of the concentration of A as a function of time yields a straight line.
When t t ½ that is the half-life of the reaction completed the concentration of the reactant A A2. A kA 0 B k0693 C 2kA 0 D k2A 0 Hard Solution Verified by Toppr Correct option is C For zero order reaction the half life period t 12 2kA 0. In the case of a zero-order reaction the rate of reaction depends on the zeroth power of the concentration of reactants.
The equation is t 12 R 0 2k. Ii The formula to calculate the half-life period of zero order reaction. The zero order reaction rate law can be represented as ratek where k is denoted as the constant rate.
T ½ A o 2k For a first order reaction A products rate kA. Welcome to allThis class i shared to chemical kinetics unit rate constant derivated for zero order reaction and half life of zero order reactionzeroorder. As for other reaction orders an equation for zero-order half-life may be derived from the integrated rate law.
Ii The formula to calculate half life period t12. Not a set value that we can calculate. The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions.
For a zero order reaction the formula is t½ Ao 2k. For the reaction given as A B A is reactant and B is a product Rate -dA dt kA0 -dA dt k dA -k dt Now Integrating both sides we get. Term half-lifeThe time required for a quantity to fall to half its value as measured at the beginning of the time period.
T ½ 1 k A o Top. The rate constant for the reaction can be determined from the slope of the line which is equal to -k. Notice that for zero-order reactions the half-life depends on the initial concentration of reactant and the rate constant.
For a first-order reaction the half-life is given by. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k For the first-order reaction the half-life is defined as t12 0693k And for the second-order reaction the formula for the half. The half-life period of zero order reaction A product is given by.
To find the half-life for a zero order reaction the equation t12 A0 2k is used. The Half-Life of a Reaction. For a zero-order reaction the mathematical expression that can be employed to determine the half-life is.
Unlike a first-order reaction in a zero- or second-order reaction the half-life is dependent on the initial concentration ie. What is the zero order rate law. A -kt c Where c constant of integration At time t 0 A A0.
For a first order reaction t½ 0693 k and for a second order reaction t½ 1 k Ao. Questions Using the integrated form of the rate law determine the rate constant k of a zero-order reaction if the initial concentration of substance A is 15 M and after 120 seconds the concentration of substance A is 0. The decomposition of NH 3 on a tungsten W surface is a zero-order reaction whereas on a quartz SiO 2 surface the reaction is first order.
The half-life of a zero-order reaction the formula is given as t12 R02k The half-life of a first-order reaction is given as t12 0693k The half-life of a second-order reaction is given by the formula 1kR0. Equations for Half Lives. The zero order kinetic rate law can be shown as below A A 0 k t ------ 1 Where A current concentration A 0 initial concentration k reaction constant t time To determine half-life dividing equation 1 by 2 t 12 A 0 2 t.
The rate constant for a zero-order reaction is measured in molL -1 s. From the slope of the line for the zero-order decomposition we can determine the rate constant. 631 See Also Rate Law Half-Lives Half-life first order.
Graphical relations and half lives. Thus for zero order reaction the half life period is directly proportional to the initial concentration. Answer i Arrhenius equation is k AeE aRT or lnk lnA RT E a.
Where k is the temperature-dependent reaction rate constant t 12 is the half-life A 0 is the initial concentration References ChemWiki Whitten et al. T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2. Slope k 131106 molLs slope k 13110 6 mol L s Figure 3.
Half life in zero order reaction Half life means 50 percent of reactants disappear in that time interval. Half-Life of a Chemical Reaction. Converting a half life to a rate constant.
Half-life or t½ is the time that elapses before the concentration of a reactant is reduced to half its initial value. A_t ktA_0 For a half-life tt_frac12 and A_tfrac12A_0 Substituting into the integrated rate law. And we typically use the concept of half-life to for example determine the age of ancient artifacts or predict when a radioactive sample will be safe to handle.

Zero Order Reactions Study Material For Iit Jee Askiitians

Half Life Expressions Chemistnate

Ppt Summary Of The Kinetics Of Zero Order First Order And Second Order Reactions Powerpoint Presentation Id 545041

Derive The Integrated Half Life Equation For Zero Order Reaction Chemistry Chemical Kinetics 12889537 Meritnation Com

Half Life Of Zero Th 0th Order Reaction Derivation Youtube